MR CLEAN NOIV Study design
Design
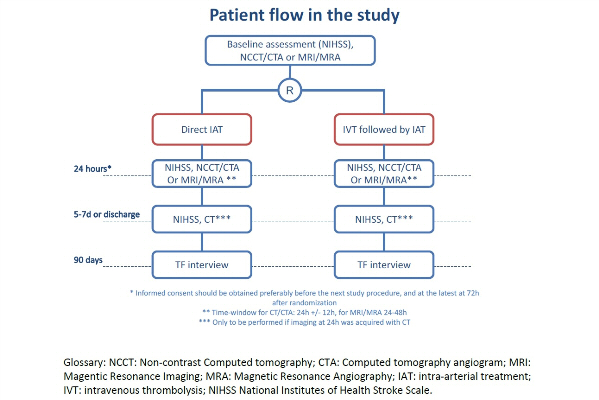
The MR CLEAN-NO IV trial is a phase III prospective randomized clinical trial with open-label treatment and blinded outcome assessment (PROBE). The study will run for 4 years in multiple intervention centers in Europe.
Population
The study population will consist of adult patients who meet the following inclusion criteria:
| Inclusion criteria |
| a clinical diagnosis of acute ischemic stroke |
| caused by a large vessel occlusion of the anterior circulation (distal intracranial carotid artery or middle (M1/proximal M2) cerebral artery confirmed by neuro imaging (CTA or MRA) |
| CT or MRI ruling out intracranial hemorrhage |
| eligible for IVT (within 4.5 hours after symptom onset) |
| a score of at least 2 on the NIH Stroke Scale |
| age of 18 years or older |
| written informed consent (deferred) |
A potential subject who meets any of the following criteria will be excluded from participation in this study:
| Exclusion criteria |
| Pre-stroke disability which interferes with the assessment of functional outcome at 90 days, i.e. mRS >2 |
| Participation in trials other than current (or MR ASAP for participating centers in the Netherlands) |
| Any contra-indication for IVT, according to national guidelines, which are in accordance with guidelines of the American Heart Association, i.e.: o arterial blood pressure exceeding 185/110 mmHg o blood glucose less than 2.7 or over 22.2 mmol/L o cerebral infarction in the previous 6 weeks with residual neurological deficit or signs of recent infarction on neuro-imaging o recent head trauma o recent major surgery or serious trauma o recent gastrointestinal or urinary tract hemorrhage o previous intracerebral hemorrhage o use of anticoagulant with INR exceeding 1.7 o known thrombocyte count less than 100 x 109/L o treatment with direct thrombin or factor X inhibitors o treatment with therapeutic dose of (low-molecular weight) heparin. |
Inclusion in other intervention trials during the study period is not allowed. Note that preceding inclusion in the Multicenter Randomized trial of Acute Stroke Treatment with a nitroglycerine patch (MR ASAP) is not an exclusion criterion for participating centers in the Netherlands.
Randomization
Patients will be randomly allocated to IVT followed by IAT (standard care) or direct IAT by a computer- and web-based procedure.
Primary outcome
The main study outcome is functional outcome at 90 days (± 14 days) as measured on the modified Rankin Scale score.
Informed consent
This study evaluates the influence of an acute treatment in an emergency situation concerning a life-threatening disorder. For every hour delay, the absolute benefit of treatment (probability or recovery to independent living) decreases by 6%. Treatment should therefore be started as soon as possible. Since most patients with acute neurological deficits are not capable of decision making, and to reduce treatment delays, the MR CLEAN-NO IV trial uses a deferred informed consent procedure, in line with Dutch law (Dutch Medical Research (Human Subjects) Act: 6,4). This implicates that patients are randomized and treated before consent is obtained. Written informed consent should be obtained within 24 hours after enrolment. This was approved by the Medical Ethics Committee of the Erasmus University Medical Center Rotterdam.