Summary
In Western Europe and the US, the annual incidence of ischemic stroke is about 2 per 1000. Half of all patients with stroke die or remain severely disabled. Treatment with intravenous (IV) alteplase, aiming at early reperfusion has been proven effective for these patients, when they are treated within 4.5 hours, and when there are no contra-indications. In about one third of the patients with acute anterior circulation ischemic stroke, symptoms are caused by a proximal occlusion of one of the major intracranial arteries. IV alteplase only leads to recanalization in up to 33% of these patients. In those without recanalization, functional outcome is generally poor.
The results of MR CLEAN trial and 4 other international randomized controlled trials on endovascular treatment (EVT) in proximal anterior circulation stroke were published (EXTEND-IA , ESCAPE , SWIFT-PRIME and REVASCAT ), all of them confirming the safety and efficacy of EVT and its importance for the advancement of stroke care. They were followed by PISTE and THRACE, both confirmed the effect of thrombectomy. This has resulted in a strong momentum swing, with clinicians and researchers worldwide now targeting ways to further improve EVT effectiveness. American and Canadian guidelines have been updated stating that EVT should be standard of care for eligible patients (AHA/ASA and Canadian). With proof of a beneficial effect available, EVT has been fully implemented and integrated in the Dutch health care system.
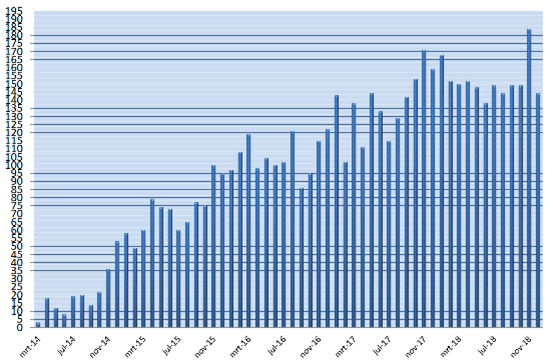
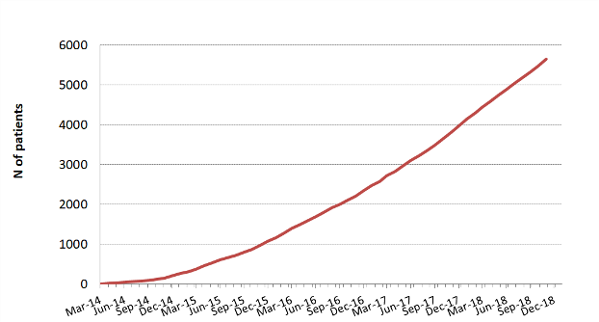
The aim of the MR CLEAN Registry was to monitor this implementation and safety of EVT in the Netherlands. All patients undergoing EVT were registered, which included patients treated in centers that started up after the MR CLEAN trials ended, and patients treated outside current indications.
The MR CLEAN Registry was a prospective national multicenter registry. 17 hospitals in the Netherlands started registering patients from March 2014 until 31 December 2018.