Study design
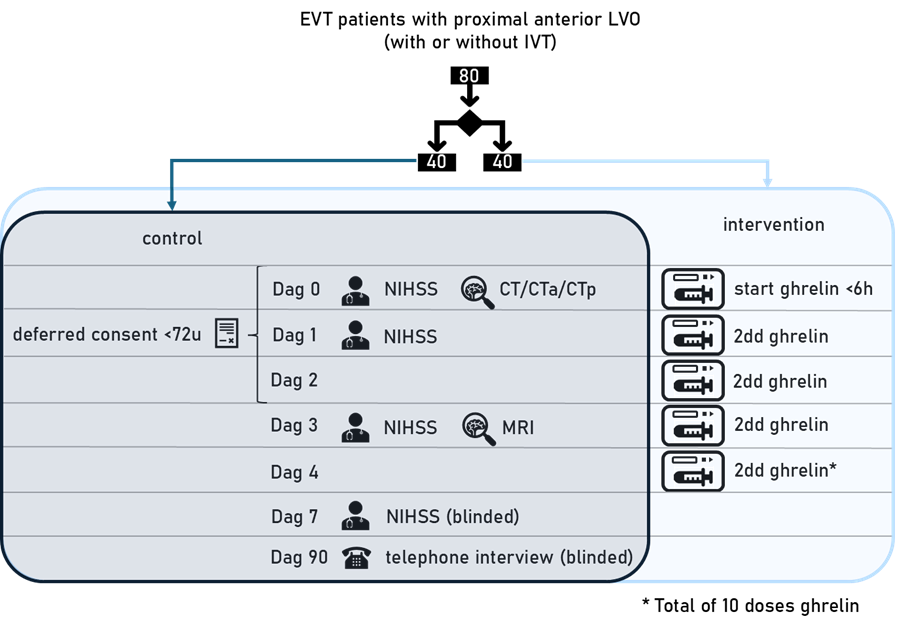
MR GENTLE is an investigator-initiated, phase 2, randomised clinical trial with open-label treatment and blinded endpoint assessment in eighty patients with acute ischemic stroke in the anterior circulation treated with EVT and a pre-EVT NIHSS score ≥ 10 in four centres in the Netherlands. Patients are randomised to intravenous acyl-ghrelin, 600µg twice daily for five days, started within six hours after stroke onset, in addition to standard care or to standard care alone. The primary outcome is the NIHSS score on day 7, adjusted for baseline characteristics. Secondary outcomes include infarct size on MRI (day 3), adverse events, and functional recovery at 90 days. Informed consent is obtained within 72 hours following randomization through a deferred consent procedure.
Study overview: